What We Do
Overview
What We Are Working On
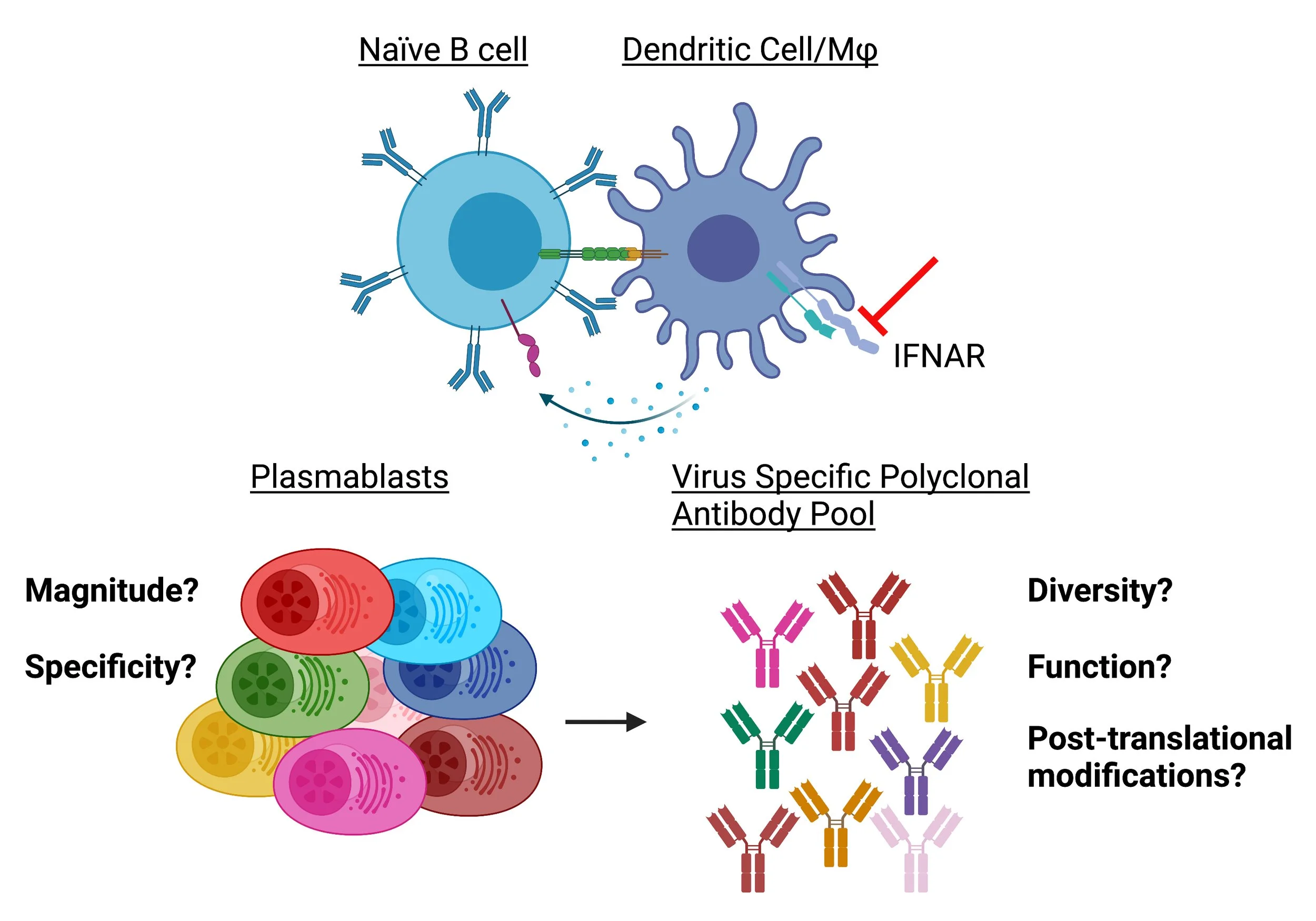
Plasmablasts are early- short lived antibody secreting cells (ASCs) that produce the first wave of virus-specific antibodies that exhibit anti-viral functions and have the ability to be class switched. During acute viral infection, production of type I interferons (IFNs) establishes the “host antiviral state” by exhibiting potent anti-proliferative and anti-viral effects, in addition to inducing expression of interferon stimulated genes (ISGs) which also play important roles in limiting viral spread.
However, most viruses can dysregulate the host type I interferon response, but it is largely unknown how a virus’s ability to antagonize the type I IFN signaling cascade alters the plasmablast response during acute infection. These studies center on how type I IFN signaling in Dendritic Cells (DCs) and Monocytes regulates the plasmablast response. We hypothesize that inhibition of type I IFN signaling during acute viral infection is advantageous to the plasmablast response by increasing the magnitude, diversity, and mechanisms of antibody-mediated protection. To challenge this hypothesis, we utilized interferon αβ receptor 1 (Ifnar1) deficient mice which globally lack Ifnar1 from the Ifnar1/2 receptor, making these mice unable to respond to type I IFNs in addition to DC and Monocyte specific knockouts of Ifnar1, CD11c X Cre Ifnarfl/fl and LysM Cre X Ifnarfl/fl, respectively. Data from these studies will help shape our current understanding of how a virus’s ability to antagonize the host type I IFN system regulates humoral immune responses.